JAJU873 August 2020
- 概要
- リソース
- 特長
- アプリケーション
- 5
- 1 System Description
- 2System Overview
- 3Hardware, Software, Testing Requirements, and Test Results
- 4Design and Documentation Support
- 5About the Author
1.1 Medical Respiratory Systems
There are several instances where it may become medically necessary for machines to assist with patient respiration, and these machines may require motors and valves depending on the specific application. For example, ventilators use motors to generate pressurized air and a system of valves to either deliver pressurized air or release air in the lungs back to the atmosphere to mechanically assist respiration. Ventilators can also be coupled with anesthesia delivery systems to keep the patient in a safe and anesthetized state. Anesthesia delivery systems incorporate numerous valves for drug mixing and patient protection. Ventilators are typically used in hospital, institutional, transport, and home environments.
There are three basic drive mechanisms in a ventilator system: bellows, piston, and turbine. For a bellows system, typically the air compression of a bellows is from a pneumatic force controlled by servo valves. For a piston system, typically the air compression is achieved from a BLDC or DC servo motor to move a piston. For a turbine system, typically a BLDC motor is used to drive a turbine (blower).
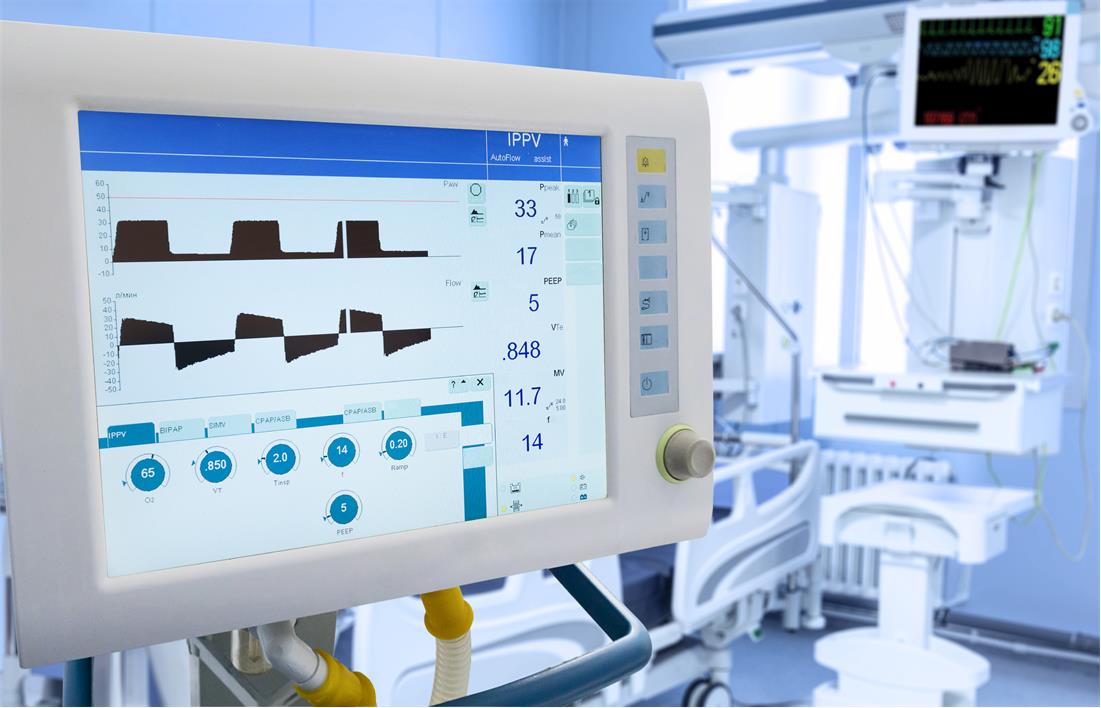 Figure 1-1 Ventilator system showing patient
vital parameters.
Figure 1-1 Ventilator system showing patient
vital parameters. The key system requirements for a ventilator system are shown in Table 1-1.
| KEY REQUIREMENTS | TYPICAL |
|---|---|
| System reliability and functional safety | IEC 60601-1, ISO 80601-2 |
| Accurate sensing of key parameters | flow, pressure, respiration rate, FiO2, temperature, and humidity |
| Power source | AC or DC |
| Efficient system design for portable systems | 8-10 hours of continuous use on battery |
| DC power input voltage | 6-28 V |
| Input power | 150-200 W |
| Operation temperature range | 5-40 °C |
| Operating pressure range | 0 – 90 cmH2O |
| Operating flow range | 0-200 L/m |
| Flow accuracy | ± 5 L/min or 20% of reading |
| Pressure accuracy | ± 0.5 cmH2O or 10% of reading |
| Minimum inspiratory/expiratory time | 200 ms |
| Valve response time | < 5ms |
| Motor operating voltage | 24 V |
| Motor operating current | 3-5 A continuous |
| High speed operation | 40-60 kRPM |
| Wide operating speed range | 1-60 kRPM |
| High accelerations and braking | 150-200 kRPM/s |
Note, many medical ventilators will also support continuous positive airway pressure (CPAP) operating mode and include an oxygen concentrator (for example, Ventec VOCSN, Medtronic Puritan Bennett™ 980). However, there are many standalone CPAP machines (for example, Philips DreamStation, ResMed AirMini™) and oxygen concentrators used for home healthcare. For CPAP machines, the pressurized air is delivered to sleeping patients through a mask to treat sleep apnea by helping to prevent the throat from closing.
Similar to ventilators, many CPAP machines and oxygen concentrators rely on a BLDC motor in their application to pressurize the air due their reliability, efficiency, and audible noise characteristics. In general, these standalone machines are designed to be portable and many weights less than 10 lbs. Standalone CPAP machines have less stringent motor requirements compared to ventilators. In addition, CPAP machines typically specify a lower sound level (~29 dBA) compared to ventilators (~50 dBA) and they have a narrower operating pressure range compared to ventilators.
The key system requirements for a CPAP system are shown in Table 1-2.
| KEY REQUIREMENTS | TYPICAL |
|---|---|
| System reliability and functional safety | IEC 60601-1, ISO 80601-2 |
| Accurate sensing of key parameters | Flow, pressure, temperature, and humidity |
| Power source | AC or DC |
| Efficient system design for portable systems | 8-12 hours of continuous use on battery |
| DC power input voltage | 6-28 V |
| Input power | 30-80 W |
| Operation temperature range | 5 to 35 °C |
| Operating pressure range | 4-20 cmH2O, sensor range 0-40 cmH2O |
| Operating flow range | 0-150 L/min |
| Flow accuracy | ± 1.5 L/min or ± 2.7 % of reading (ISO 80601-2-70) |
| Static and dynamic pressure accuracy | ± 0.15 , ± 0.27 cmH2O (ISO 80601-2) |
| Sound level / noise emissions | <29 dBA (ISO 4871) |
| Service life | 5 years |
| Physical weight | 1-4 lbs |
| Motor operating voltage | 12 or 24 V |
| Motor operating current | 2-3 A continuous |
| High speed operation | 30-40 kRPM |
| Wide operating speed range | 1-40 kRPM |
Standalone oxygen concentrators will similarly use a motor to pressurize air and will use multiple valves to mix air with pure oxygen to increase the oxygen concentration in the mixture delivered to the patient. One of the key differences in requirements compared to the previous applications is the lower motor speed (2-4 kRPM) due to a lower flow rate requirement (typically less than 2 L/min).
The key system requirements for an oxygen concentrator are shown in Table 1-3.
| KEY REQUIREMENTS | TYPICAL |
|---|---|
| System reliability and functional safety | IEC 60601-1, ISO 8359, ISO 80601-2-67 |
| Accurate sensing of key parameters | Flow, pressure, FiO2, temperature, and humidity |
| Power source | AC or DC |
| Efficient system design for portable systems | 4-10 hours of continuous use on battery |
| DC power input voltage | 10-28V |
| Input power | 100-150W |
| Operation temperature range | 5 to 35 °C |
| Maximum outlet pressure | 30 to 90 psi |
| FiO2 Range | 21-100 % |
| O2 sensor accuracy | 3-6 % |
| Operating flow range | 0-10 L/min |
| Flow accuracy | +/- 15 % |
| Sound level / noise emissions | 40 dBA |
| Service life | 5 years |
| Physical weight | 5-10 lbs |
| Motor operating voltage | 12 or 24 V |
| Motor operating current | 2-5 A continuous |
| Motor speed | 2-4 kRPM |